IACUC Review Flowcharts
IACUC Review Process Overview
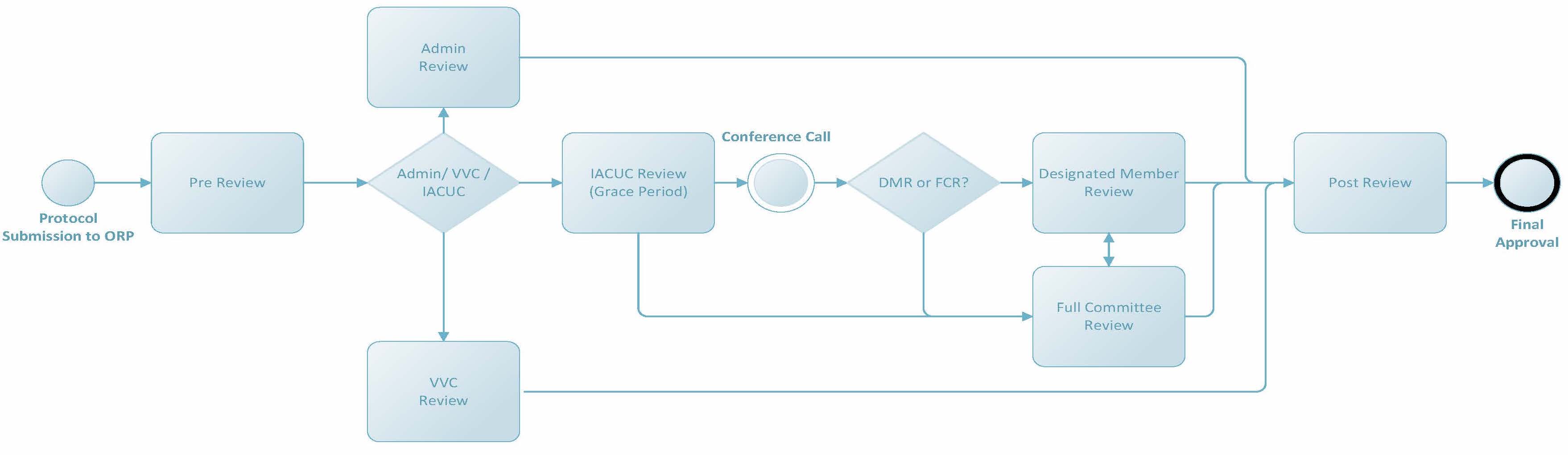
For more details about a specific part of the process, refer to the appropriate link below. More complex parts are further broken down to give a comprehensive overview of the various review processes and steps.
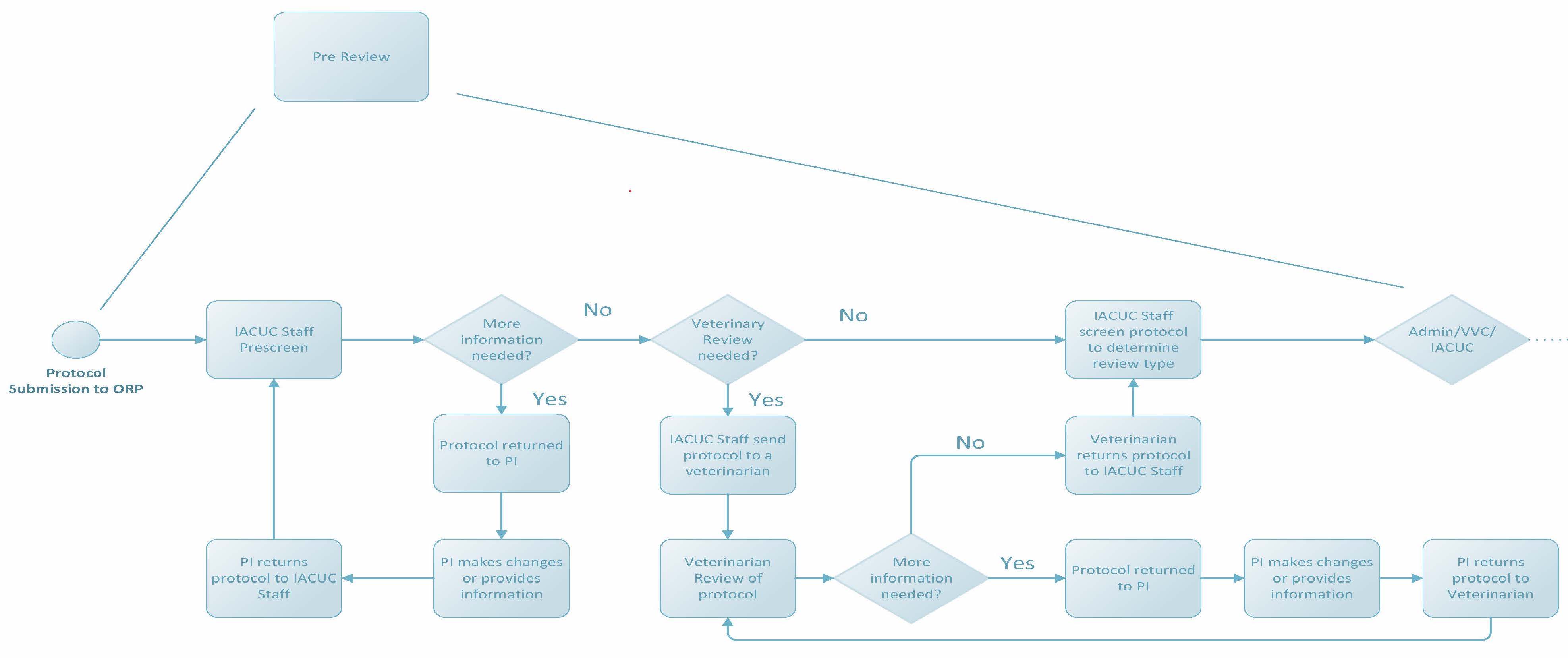
Pre Review
Once a protocol is submitted to the Office for Research Protections, it undergoes a thorough pre-review process. IACUC staff will review protocols for clarity and completeness following protocol review standards required by applicable federal and state regulations. Staff may enter comments into protocols within CATS IACUC and return the protocols to researchers to edit or provide clarification if needed. Required trainings (i.e. CITI courses) and ancillary reviews (such as biosafety or human research) will be assigned by staff, and researchers will be contacted to address outstanding requirements. For protocols that involve painful and/or distressful procedures like surgery, a veterinarian must be consulted in the planning of such procedures. This “Vet Consult” also occurs during the pre-review process
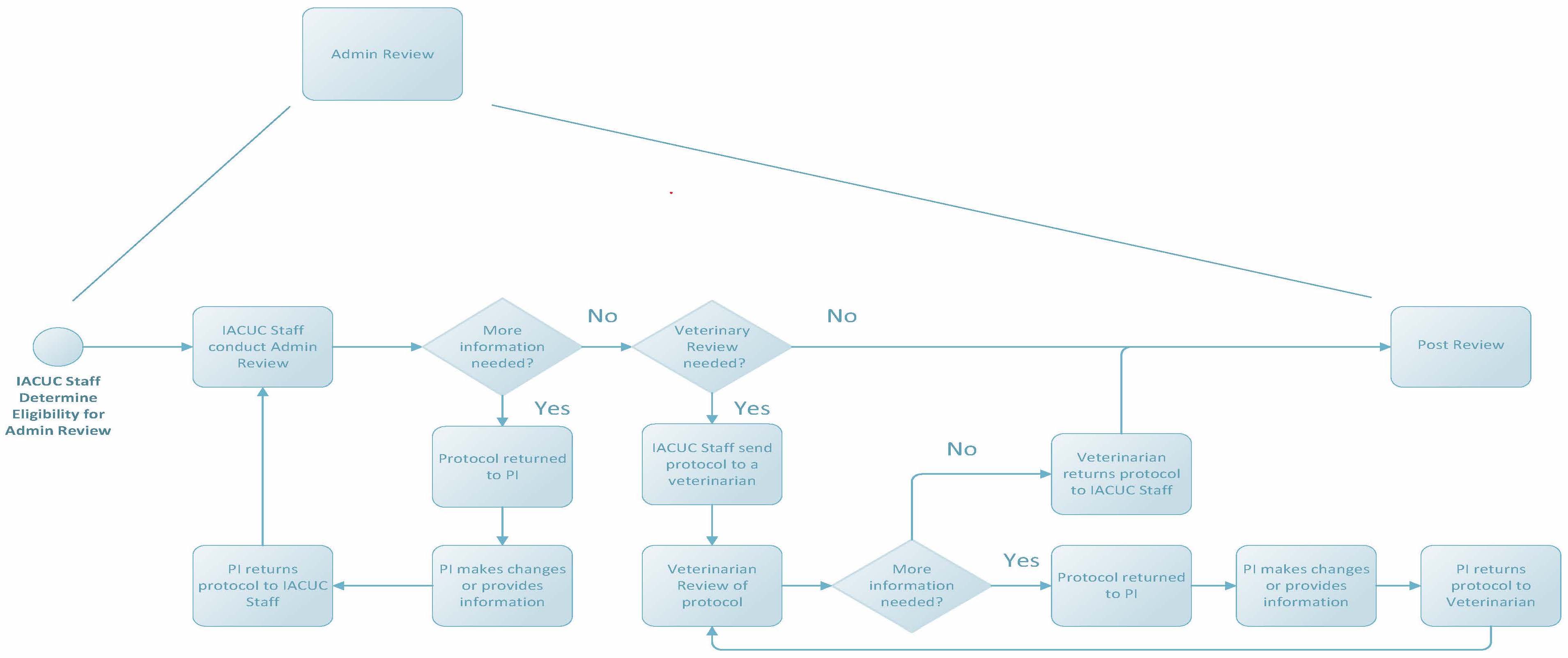
Administrative Review
Federal regulations allow some protocol changes to be reviewed administratively as described by internal policies and procedures. Changes that can be reviewed administratively by IACUC staff or veterinarians include, but are not limited to, the following: change in protocol title, change in funding source, change in personnel (other than the PI), addition of a new strain, and some Annual Reviews. Submissions that must be sent to the IACUC for review include components like a change in PI, change in species, or an increase in the degree of invasiveness of a procedure or discomfort to an animal.
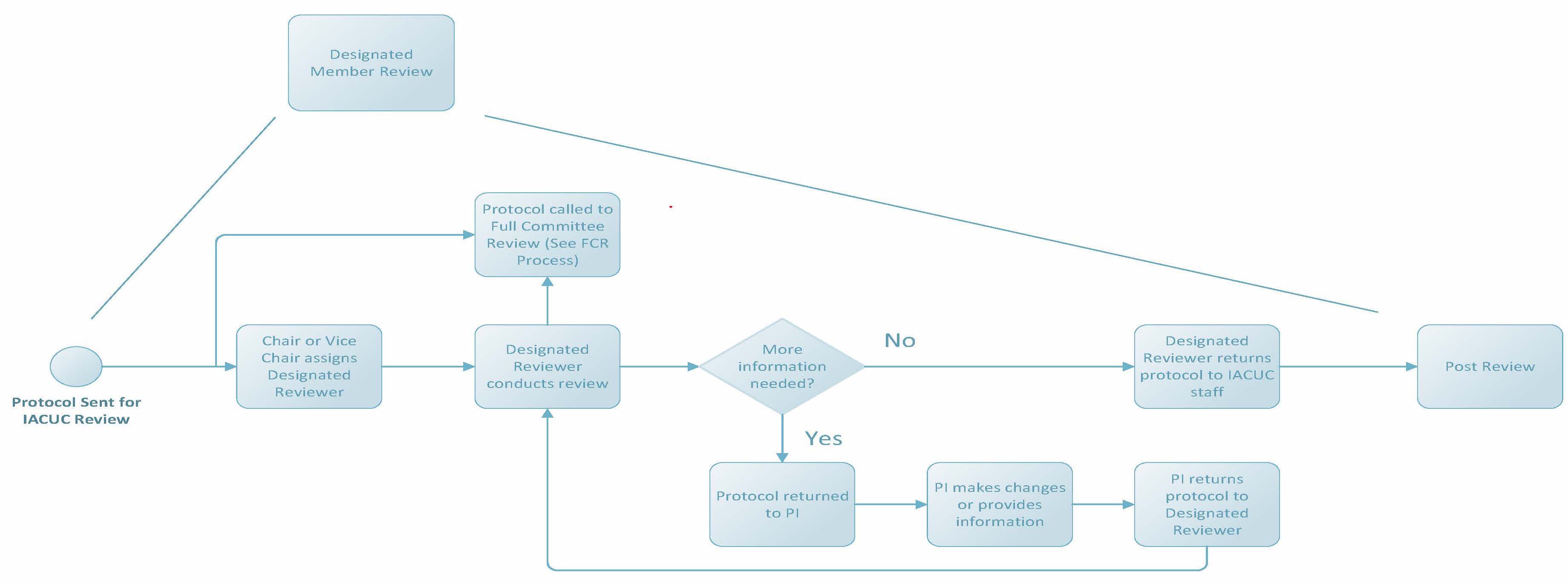
Designated Member Review
If IACUC staff determine a protocol cannot be reviewed administratively or by VVC, the protocol will be submitted to all IACUC members for review. The Public Health Service Policy on Humane Care and Use of Laboratory Animals (PHS Policy) requires that all IACUC members have access to review a protocol and can request full committee review (FCR) if needed. The period of time for member review at Penn State lasts about 10 calendar days and protocols will display the review state of “Grace Period” in the protocol management system CATS IACUC. Upon conclusion of the grace period, if FCR is not requested, the IACUC Chair or Vice Chair will designate at least one member of the IACUC (designated member review, or DMR) to conduct the review. This designated reviewer has the authority to approve, require modifications to (to secure approval), or request full committee review of a protocol.
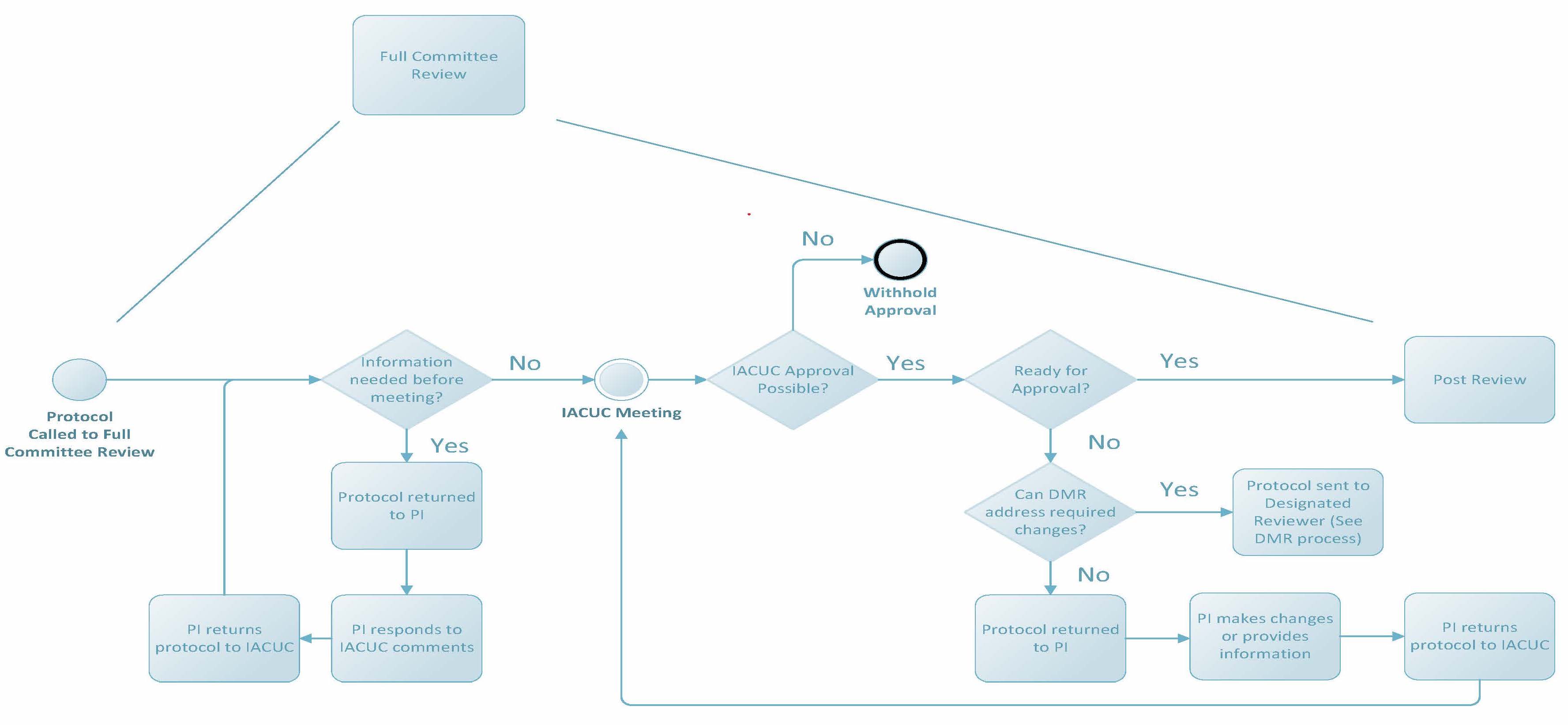
Full Commitee Review
Per the PHS Policy, if full committee review (FCR) is requested, approval of the protocol may be granted only after review at a convened meeting of a quorum of the IACUC and with a majority vote to approve. Prior to review at a convened meeting, the IACUC may request additional information from the researcher and/or revisions to the protocol to address minor omissions or needs for clarification. Once the protocol is reviewed at a convened meeting, the IACUC may vote for one of the following determinations: Approve, Withhold Approval (reasons will be provided to the PI in writing), Require Modifications to Secure Approval (following revisions, the protocol will return to the IACUC at an upcoming meeting), or have the Chair or Vice Chair assign the protocol to a designated member reviewer subsequent full committee review (DMR sub FCR) to address any remaining concerns. This last determination requires a unanimous vote; if the vote for DMR sub FCR is not unanimously in favor of the motion, the protocol cannot be reviewed following this mechanism.
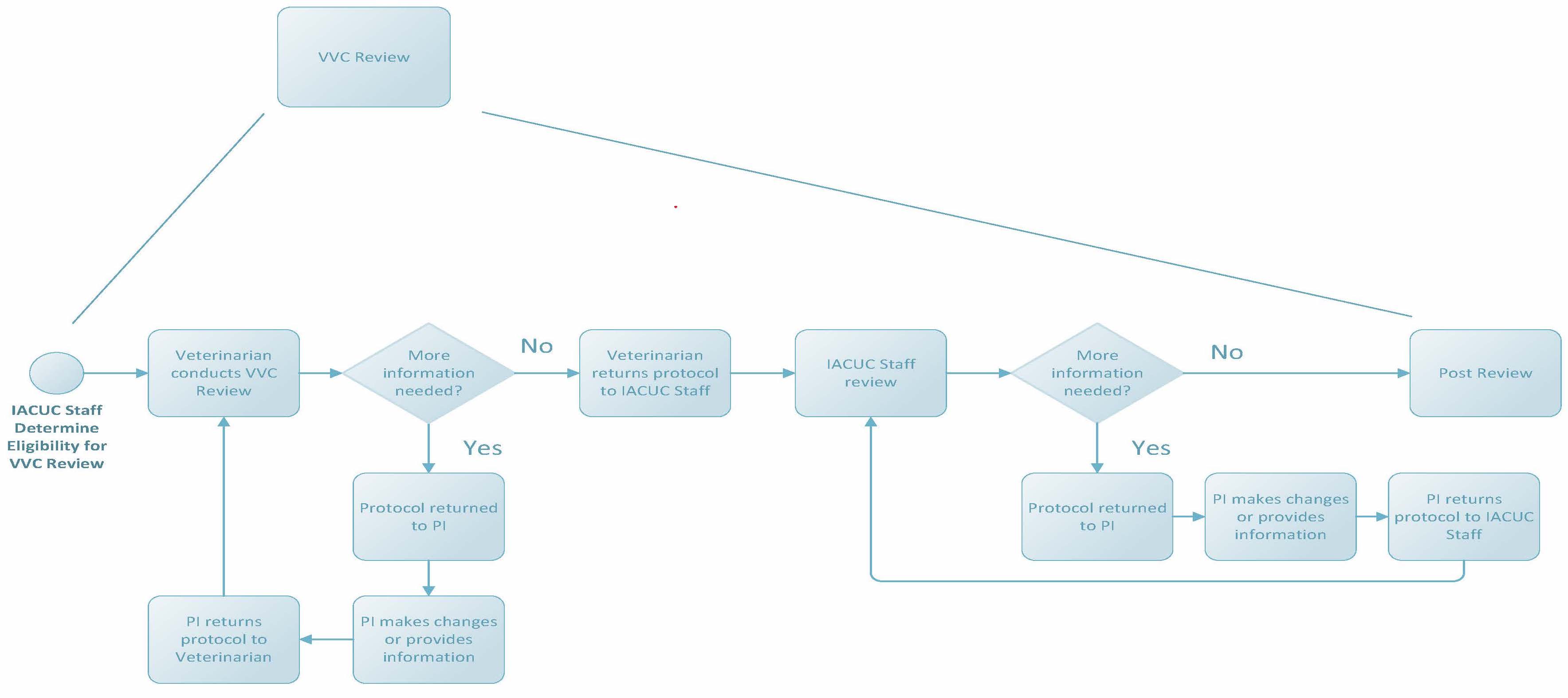
VVC Review
In August 2014, the National Institutes of Health (NIH) released a notice titled “Guidance on Significant Changes to Animal Activities”. This notice provides guidance to IACUC’s on significant changes and the allowable review mechanisms for specific changes. Specific significant changes as outlined by the notice may be handled administratively according to IACUC-reviewed and -approved policies in consultation with a veterinarian authorized by the IACUC. See IACUC Policy 13 for more information and examples of significant changes that may be reviewed by VVC. Protocol submissions reviewed by VVC will follow a review flow path like that of pre-review, with comments entered by a veterinarian and returned to researchers for revisions and clarifications as needed. Note: the veterinarian may still refer any request to the IACUC for review for any reason.
Post Review
Once the reviewer (IACUC staff, veterinarian, designated member, or full committee) has granted approval to the protocol within the CATS IACUC system, the protocol enters the state of “Post Review”. In this state, IACUC staff will review the protocol for outstanding requirements such as trainings, ancillary reviews, inspection of equipment or facilities, submission of permits or other requested documentation, etc. Once all requirements needed for approval are met, IACUC staff will draft and send the final approval letter.
