Office for Research Protections | Institutional Review Board
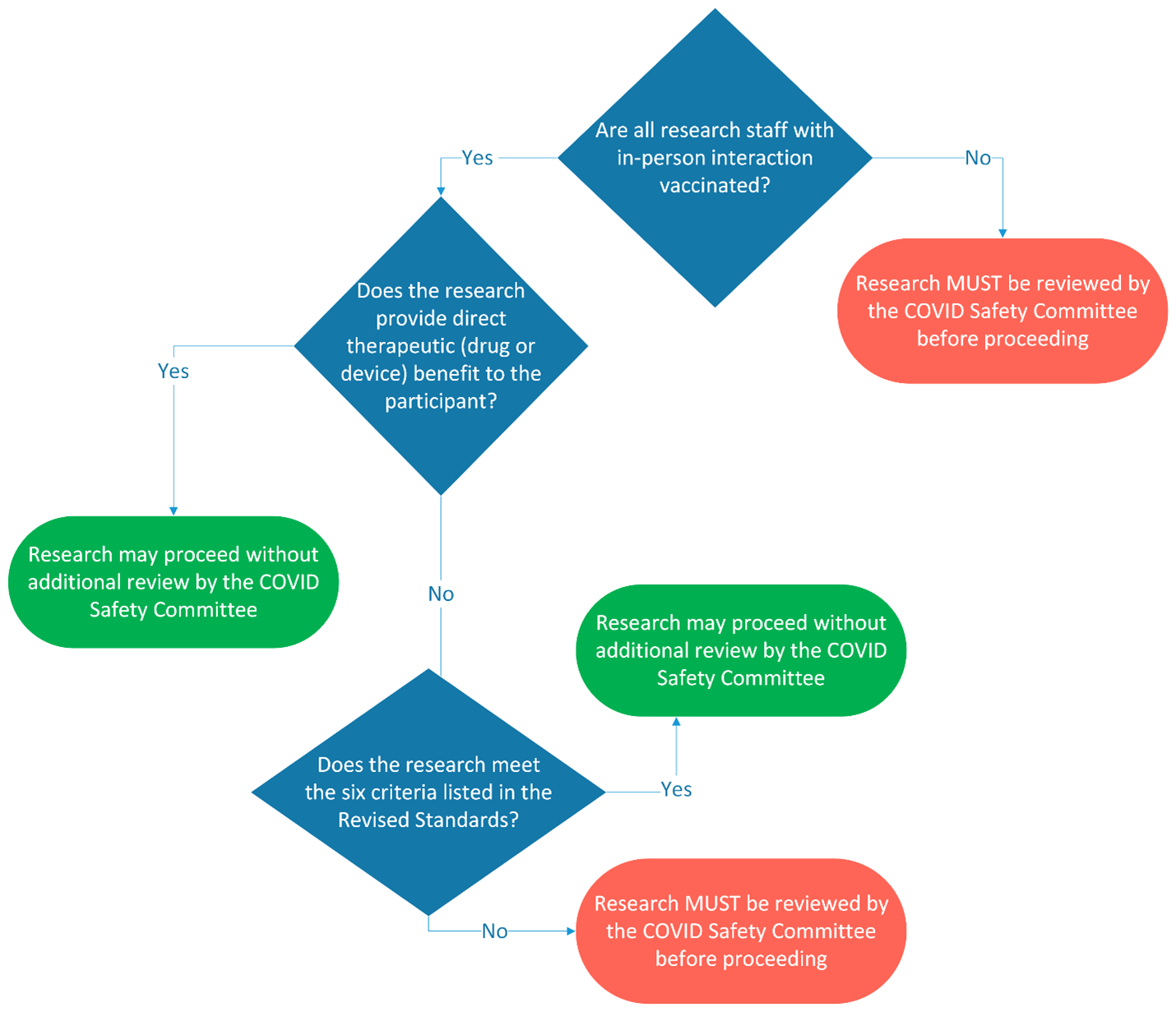
Dr. Lora Weiss, senior vice president for research, in consultation with Dr. Kevin Black, interim dean of the College of Medicine, announce an update to the Revised Standards for in-person human subjects research. Effective October 1, the Revised Standards are applicable to ALL studies with an in-person research component. Previously, human subjects research considered potentially therapeutic (drug or device studies) were exempt from the Revised Standards. Moving forward, all study personnel with in-person contact with research participants must be fully vaccinated OR the study must be reviewed by the COVID Safety Committee. In addition, studies previously approved through InfoReady will also need to resubmit if study team members with in-person interaction are not fully vaccinated.
Studies that previously did not require review have until October 15 to submit to the COVID Safety Committee. Studies will be reviewed by their college, followed by the University COVID Safety Committee. Studies that did not originally require review or were previously approved may continue while they are under review. It is important to note that this review is separate from the IRB review and focuses solely on conducting research as safely as possible during the pandemic. Learn more about the Procedures to request to conduct in-person research.
For research with a direct therapeutic (drug or device) benefit:
-
If all research staff with in-person participant interaction are FULLY VACCINATED, research may proceed without additional review by the COVID Safety Committee.
-
If any research staff with in-person participant interaction are NOT fully vaccinated, research MUST receive approval from the COVID Safety Committee via InfoReady before proceeding. It is the expectation of the committee that participant interaction with non-vaccinated staff will be eliminated or minimized.
For observational research (no direct therapeutic benefit):
-
If the in-person, human subjects research meets the six criteria outlined in the Revised Standards, it may resume WITHOUT additional review and approval by the COVID Safety Committee via InfoReady.
-
In-person, human subjects research that does not meet criteria 1-6 in the Revised Standards MUST SUBMIT through InfoReady for review by their college dean/chancellor and the COVID Safety Committee in the Office of the Senior Vice President for Research.