Ancillary Review
Ancillary review assists the primary review body with matters related to research feasibility, risk, regulatory requirements, and research compliance. Not all studies require ancillary review. During IRB review, staff of the IRB office will manually select the reviewer or reviewing organization/department each time a review is needed or required. IRB staff can add ancillary reviewers to a study, modification, or continuing review. See "HRP-309: Ancillary Review Matrix" in the CATS IRB Library for general guidance on ancillary review types and how the ancillary review impacts IRB review and approval.
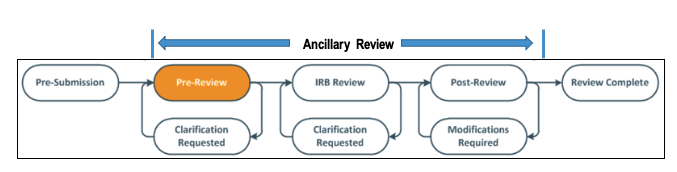
Ancillary review determinations can be recorded at any time from the Pre-Review to Post-Review states, as illustrated below.
All researchers must comply with any policy that requires an ancillary review in order to proceed with IRB review and approval. The following chart provides some examples of ancillary reviews that require a separate submission to a different review body. See "HRP-309: Ancillary Review Matrix" in the CATS IRB Library for more detailed information.
|
|
|
|
|---|---|---|
| IACUC | Research involving vertebrate animals | Complete an IACUC application and obtain an IACUC number |
| IBC | Research involving biohazardous materials (defined in PSU policy RP11) | Complete an IBC application and obtain an IBC number |
| Conflict of Interest (COI) | Research studies in which a member of the study staff has a financial interest or institutional conflict as defined by PSU policies | Submit a recommended management plan |
| Unmanned Air Systems | Research that will involve the use of Unmanned Air Systems | Submit a flight request to and receive approval from the UA Operations Management Team |
