2023 CATS IACUC Upgrade
Upgrade Overview Video

System-Generated Emails
- CATS IACUC-generated emails will now come from noreply-iacuc@psu.edu. This email address services all Penn State locations.
- Researchers should not directly reply to this email address as the system will not deliver a reply to the IACUC Office.
- Instead, please send any inquiries to the appropriate IACUC Office listed at the bottom of the email.
Breadcrumbs
- The Breadcrumbs trail is now visible on the Submission Workspace.
- It allows researchers to easily navigate between follow-on submissions like Amendments and Continuing Reviews, the parent protocol, and the research team workspaces.
Resizing Help Text Windows
- Researchers can now resize Help Text windows by clicking on any corner or edge of the pop-up, and dragging the window to either expand or reduce its size.
- Resizing pop-ups provides the flexibility to view all the information within a Help Text window without the need to scroll the sidebar.
- When expanding, the scroll bar disappears and you’re able to view all the information in the pop-up.
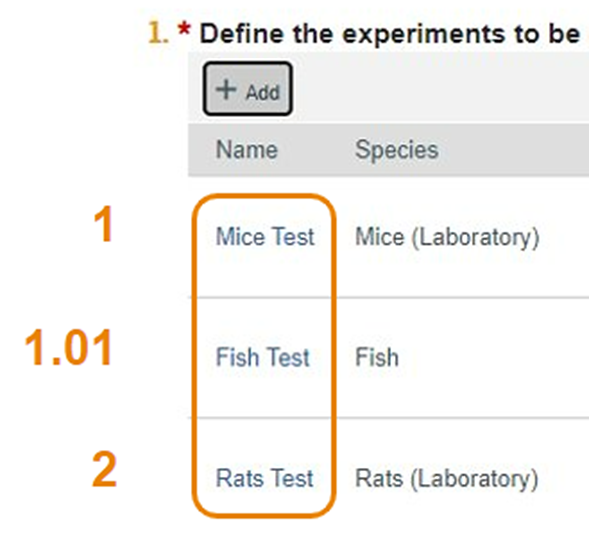
Sorting Experiments
- Researchers can now sort Experiments on the Experiments page by including a number up to two decimal points in the Display Order field.
- The new order will appear on both the Experiments page and Experiments tab.
- In the below example, the researcher has ordered the Fish Test experiment as 1.01.
- Experiments will be displayed in the order entered by the protocol team.
- Below is a snapshot of the left side of the Experiments page.
- The Mice Test was ordered with an input of 1 and the Rats Test inputted as 2.
- Remember, the Fish Test was ordered as 1.01, so it displays after the Mice Test, but before the Rats Test.
- With this upgrade, you will see the Experiment names listed, but not the ordered numbers.
- Experiments with no number in the Display Order field will order alphanumerically by name also on both the Experiments page and tab, and will be listed above Experiments that have a display order.
Previously Approved Procedures
- Procedures that are already on an approved protocol now display a check mark (✓).
- These checks will appear on the Experiments page; Experiments tab; and on the printer version.
Tissue & Blood Collection Procedures
- Tissue and Blood Collection Procedures questions are now reordered to follow a pre-procedure > procedure > post-procedure flow .
Adjusting Animal Count
Click here to watch a 2-minute video.
- In Question 1 of the Animal Justification section, the Animals Identified in Experiments column totals each species by pain category for all experiments listed on the Experiments page.
- If a researcher is reusing animals, the totals in this column do not account for this.
- However, the Adjusted Animal Count column exists to allow the researcher to account for animal reuse.
- To indicate reuse of animals across experiments, researchers can adjust the total number of animals requested by species and pain category by entering a lower value in this column.
- In the below image, the researcher plans to reuse the same 50 mice from their Pain Category C experiment in their Pain Category D experiment. Therefore, they do not need 100 total mice; they only need 50.
- They’ve adjusted the animal count to 0 in the lower pain category to account for 50 mice total on the protocol, as opposed to 100.
- They’ve maintained the animals in Pain Category D since all animals must be included in the highest pain category they may experience.
- Researchers may change the numbers in the Adjusted Animal Count column as needed to reflect the total number of animals that they truly need for the entire protocol.
Annual Review to Continuing Review
- The term Annual Review will be changed to Continuing Review which aligns with federal policy from USDA and NIH.
- Penn State will still require annual Continuing Reviews, but Annual Review will now read as Continuing Review and the prefix will now read as IACCR as opposed to AR.
-
Annual Review submissions approved prior to the Upgrade will still have an AR prefix.
Alternatives Searches & Duplication
- There are new improvements to the Alternatives Searches and Duplication page.
- This page now displays Procedures on the protocol that are designated as causing more than momentary pain or distress, so researchers know which procedures will require a search for alternatives in question #1.
- This page now includes an assurance statement that the activities described in the proposal do not unnecessarily duplicate previous research.
- PIs must affirm to this statement by checking “Yes” in order to move forward in the submission process.
Additional Topics (not in upgrade video)
Reviewer Notes
- Clicking on the Reviewer Notes tab will reveal the following sub-tabs: Comments tab, showing all reviewer notes within a protocol, and Documents tab, showing all documents attached to reviewer notes.
- Click on the Reviewer Notes tab to return back to reviewer notes.
- After approval, the Reviewer Notes tab is no longer displayed in the IACUC Submission Workspace, rather the reviewer notes summary document (PDF) is available under the Documents tab.
Field Research Assurance
- A new assurance statement has been added to the Field Research Details page of the protocol form to align with compliance of applicable local, state, national, and international wildlife regulations.
- Before conducting animal research, researchers will be required to agree with this assurance statement for all new field research protocols and for any amendments to or at the time of a triennial review of an existing field research protocol.


