IACUC Triennial Reviews
Triennial review is a complete (de novo) IACUC review of the previously approved protocol that is federally required every three years to receive continued approval of ongoing activities on the protocol. The protocol PI, PI Proxy(ies), and Primary Contact receive an email notification when a triennial review is coming due.
Begin a Triennial Review
To begin a Triennial Review, follow the instructions below:
Click on the link to the protocol workspace from the email OR open the protocol workspace following these steps:
- Log in to CATS IACUC (http://iacuc.psu.edu).
- Click Submissions in the left sidebar menu.
- Click on the Active tab.
- Click on the Name of the protocol that is due for triennial review to access the protocol workspace.
Next, select "Create Triennial Review" from the Next Steps menu.
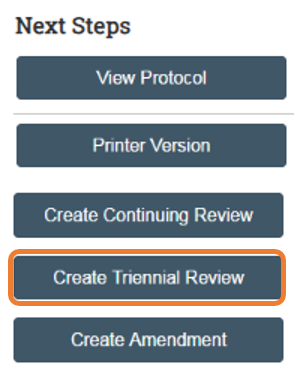
Triennial Review Summary Page
Then complete the Triennial Review Summary Page and include the following:
- a concise summary of the animal use/research activities conducted to date, including what aspects of the experiment(s) have been completed thus far
- a summary of work to be conducted in the next three years of approval. Distinguish what work has been completed, what remains yet to be done, and any changes/additions included.
- an updated animal usage count to date listed under Update Animal Use Count. Add any animal usage for your currently approved protocol that is not already listed.
Update the Protocol
Click “Continue” to open the protocol form and complete the Triennial Review submission. The image below depicts the "Continue" button at the bottom of the page.
Important!
- The Triennial Review submission will automatically populate with all responses from the currently approved protocol.
- All responses must be reviewed and updated accordingly to reflect the next three years of research/activity with special consideration to the following:
Basic Information
Update the Brief Lay Summary to summarize the next three years of research/activity.
Protocol Team Members
Add or remove personnel from your protocol as needed.
Funding Sources
Add or remove funding sources from your protocol. If no external awards fund this work, add your department or other internal funding source.
Penn State Hershey researchers: If the protocol is associated with a grant (external or internal), please attach the following sections (or comparable sections) of the final grant document: Title page (should include grant title, funding agency, funding period, grant number); Specific Aims; Research Strategy; and Vertebrate Animals Section.
Scientific Aims
Update the Scientific aims and the Significance and benefits of the research to account for the next three years of research/activity.
Personnel should review their Experience entries and update the entry in their profiles to reflect any experience and training they have received since the last entry update. Review how to update "Experience" entries.
To edit team members:
- Click the edit button next to the Tem Member as seen in question 1 below.
To edit team member eperience:
- Click the name of the team member as seen in question 2 below.
To select the team members who will perform each procedure:
- Check/uncheck boxes next to the team member's name as show in the image below.
Be sure to click "Save", "Continue", or "Exit" then the "Save Changes & Exit" button to save your changes and exit out of the pop-up.
Experiments
If an experiment is no longer needed, click the Delete (x) to remove it as shown in the two images below. For ongoing or not yet started experiments, add any new componenets to the experimental description (experiment question 3).
Update the animal numbers in each experiment to reflect the total number of animals needed for the next three years of research (experiment questions 6 and 7).
University Park and Commonwealth campus researchers: If amendment details were added to the end of the experimental description in question 3, incorporate those details into the experimental design for the next three years of approval when updating ongoing experiments.
Note re: Sorting Experiments
- Researchers can now sort Experiments on the Experiments page by including a number up to two decimal points in the Display Order field.
- The new order will appear on both the Experiments page and Experiments tab.
- In the below example, the researcher has ordered the Fish Test experiment as 1.01.
- Experiments will be displayed in the order entered by the protocol team.
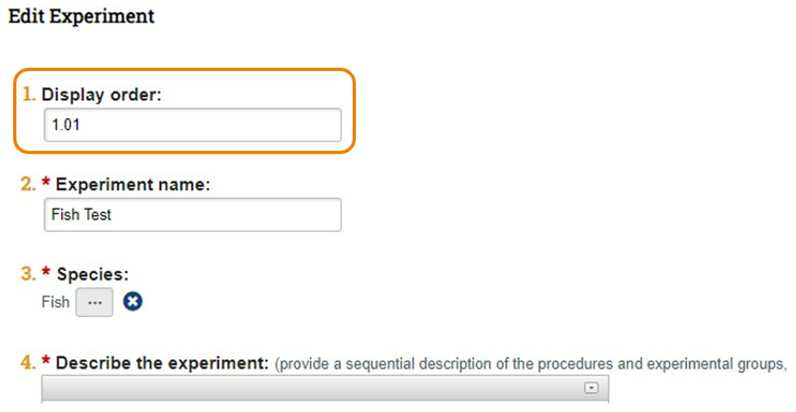
- Below is a snapshot of the left side of the Experiments page.
- The Mice Test was ordered with an input of 1 and the Rats Test inputted as 2.
- Remember, the Fish Test was ordered as 1.01, so it displays after the Mice Test, but before the Rats Test.
- With this upgrade, you will see the Experiment names listed, but not the ordered numbers.
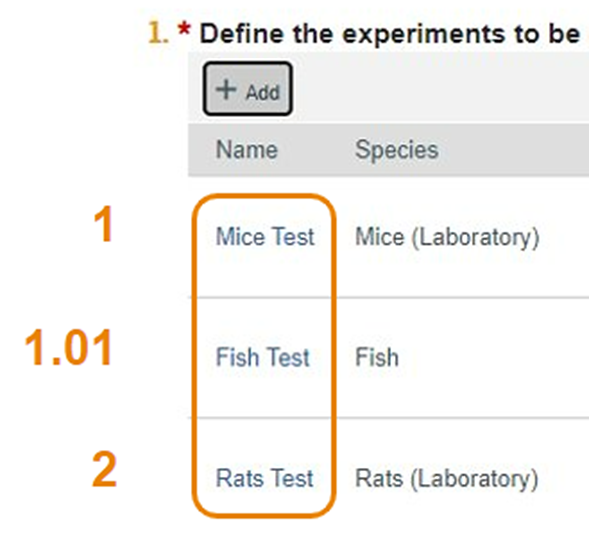
- Experiments with no number in the Display Order field will order alphanumerically by name also on both the Experiments page and tab, and will be listed above Experiments that have a display order.
Procedures
Archived Procedures:
Experiments with archived procedures must be updated by removing the outdated procedure and, if applicable, adding the new version (experiment question 6) before the triennial review can be submitted.
The triennial review workspace displays a warning indicating “A procedure associated with this protocol is archived and may have newer versions. Archived procedures must be replaced or removed before the next triennial review can be submitted or approved.”
If any procedure that is listed directly on the experiment is archived, you will see the word “Archived” at the end of the procedure name in the Procedures column on the main Experiments page, as depicted in the image below.
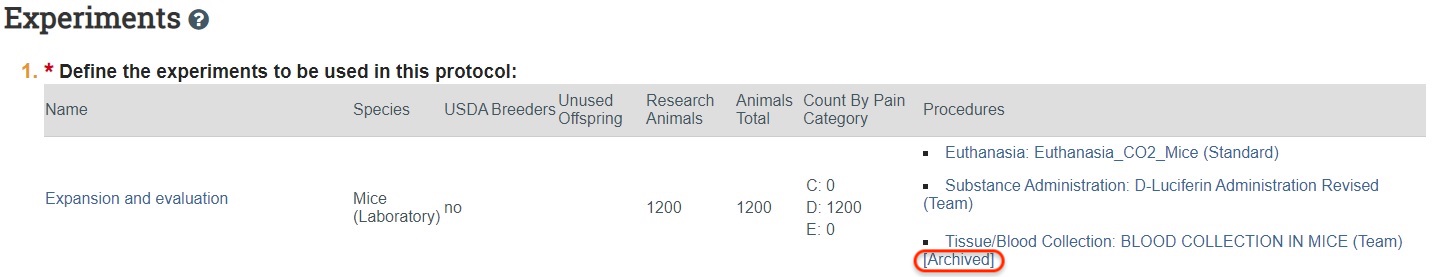
Archived procedures that are nested only (listed in the related procedures question within another procedure but were not also added directly on the experiments page) are shown by clicking “Validate” at the top of the TR form.
Updating Procedures:
If changes to procedures are needed to reflect the research/activities for the next three years (e.g., addition of new substances), create a new version or copy of the procedure. Make desired changes in the new version/copy, including removing any archived nested procedures. Learn how to create and copy a procedure or watch this video for more guidance. Remove outdated versions/copies of procedures from the experiment(s) and add the new versions to the experiment(s), including any procedures (e.g., anesthesia, analgesia) that were previously only nested (listed in the related procedures question within another procedure but were not also added directly on the experiments page). Contact your campus's IACUC office with any questions.
Note:
- The previously approved version(s) of all procedure(s) must continue to be followed on this protocol until the triennial review with updated procedures is approved.
- If a previously approved version of any procedure is listed on other approved protocols, it must continue to be followed for those protocols until/unless the updated version is added to and approved on those protocols.
- Copying vs. Versioning
- Copy Procedure: procedure changes being made are only intended for one or some (not all) protocols.
- Create New Version: all procedure changes are intended for all protocols (an amendment or triennial review must be submitted to update procedures on other approved protocols).
Personnel
Personnel should review their Experience entries and update the entry in their profiles to reflect any experience and training they have received since the last entry update. Review how to update "Experience" entries.
Strains
Remove transgenic strains you are no longer using or add strains you intend to use in the next three years.
Penn State Hershey campus researchers: Listing strains on the protocol is only required if the strains being used present with any clinical signs of concern/phenotype requiring special care that animal care/veterinary staff should be aware of (e.g., immunocompromised strains, prone to spontaneous tumor growth, or brittle bones, etc.).
Animal Justification
Click here to watch a 2-minute video.
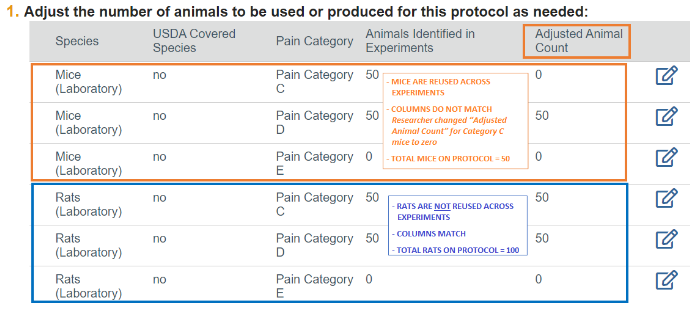
The “Animals Identified in Experiments” column automatically calculates the total for each species by pain category from all experiments listed on the Experiments page. If you will reuse the same animal(s) in more than one experiment, the totals in this column do not account for this reuse.
Thus, the “Adjusted Animal Count” column exists to allow you to account for animal reuse. The “Adjusted Animal Count” column may not reflect all experimental nuances and should be reviewed at every submission to ensure it accurately reflects the number of animals you are requesting in all experimental descriptions (i.e., number of groups and numbers per group).
To indicate reuse of animals across experiments, you can adjust the total number of animals requested by species and pain category by entering a lower value in the “Adjusted Animal Count” column. You should change the numbers in this column as needed to reflect the total number of animals that you truly need for the entire protocol.
- In the example below, the researcher only needs a total of 50 (not 100) mice for the protocol as they will reuse the same 50 mice in different experiments.
- As a reminder, all animals must be included in the highest pain category they may experience.
- Thus, they have adjusted the animal count to 0 in the Pain Category C.
If you are not reusing animals across experiments, the “Adjusted Animal Count” column and the “Animals Identified in Experiments” column should match. This indicates that no animals are being reused. You will simply type "N/A" in question 2.
The “Adjusted Animal Count” column should never be more than the animals identified in experiments. If you need to increase the number of animals for your protocol, this should be done on the Experiments page, and all related responses (e.g., animal numbers within experiments, experimental descriptions, animal justification page responses).
If reuse of animals is occurring on the protocol, you will explain the reuse in question 2 on this page.
To adjust the animal counts:
- Click anywhere in the row.
- Type the new number into the “Adjusted Animal Count” column.
- Tip: Do not click the X icon unless you want to discard your changes for the row. To collapse the edited rows, continue with the next steps.
- For the next question, type an explanation for the adjusted counts.
- Click the Save button, which will collapse the rows.
Alternatives
All literature searches for alternatives must be redone at the time of the Triennial Review. Click “Update” to the left of any outdated searches to update the search details.
- Remove/switch out archived procedures for current versions
- Date of search
- Years of the search
- Consider refining search terms and keywords used. Refer to the following resources for guidance on alternative searches:
- Researchers at Penn State Health and College of Medicine, see http://harrell.library.psu.edu/iacuc
- All other Penn State researchers, see https://guides.libraries.psu.edu/IACUC
Conduct a search for alternatives for any new procedures added to the protocol that may cause any more than momentary pain or distress.
Housing and Use
Add or remove housing and use locations from your protocol.
Disposition
Verify that animal disposition indicated is still accurate. Update if needed.
University Park and Commonwealth campus researchers: If animals are transferred to another protocol, verify the protocol information provided in question 2 is still correct. Update if needed.
Breeding
Review the details of the genotyping procedure(s) listed in question 3; select the genotyping methods you will use. If no longer current, replace the listed procedure with an updated procedure.
In question 4, check "Yes" if strain(s) listed on the protocol will require delayed weaning per the appropriate institutional guidance (HY: >21 days; UP: >26 days). Refer to the blue Help Text bubble next to question 4 for more information.
Field Research
- Update field locations as appropriate.
- Verify that contingency plan(s) for injured animals are still correct.
- Review and/or update required permits to conduct the field research and add to Supporting Documents if possible.
- Given the target species and non-target species that may have been caught/observed in the past three years, review the biological and ecological impacts of the research and update as appropriate.
Update any outdated documents.
- Penn State Hershey campus researchers: Attach an updated IACUC Protocol Snapshot. If needed, a blank Protocol Snapshot form may be found in the Related Forms page of CATS IACUC. If no information has changed, simply update the D it was last reviewed.
- All other Penn State researchers: If your protocol includes an Animal/Biohazard form (see the Support Documents page help text for clarification), an updated version to cover the next three years of research/activity is required.
Submit a Triennial Review
Once finished with all changes, click “Save and Exit” to close the protocol and save changes. Click "Submit"in the Next Steps menu for IACUC review.
