Rodent Handling and Identification
How to Restrain a Mouse
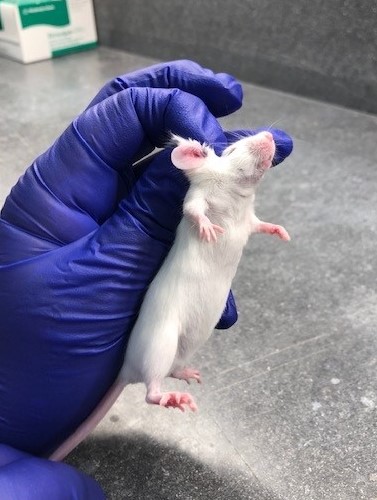 The three-finger restraint technique is a refinement of the traditional method for scruffing mice. The traditional method, which utilizes two fingers, creates a longitudinal skin fold along the mouse’s neck. This fold places pressure over the throat and trachea, causing discomfort and stress to the animal. Electrocardiography measurements of mice scruffed by the traditional method have detected severe bradyarrhythmias during immobilization which persist for several minutes after restraint. These effects on the heart rate and rhythm were not seen with the three-finger method. The three-finger restraint is achieved by gripping the skin fold over the scruff with the thumb and middle finger, then substituting the middle finger with the index finger, thus creating a transverse skin fold along the neck. The transverse fold across the neck removes the pressure from the throat area. A short video demonstrating this restraint method may be found on the Norecopa website.
The three-finger restraint technique is a refinement of the traditional method for scruffing mice. The traditional method, which utilizes two fingers, creates a longitudinal skin fold along the mouse’s neck. This fold places pressure over the throat and trachea, causing discomfort and stress to the animal. Electrocardiography measurements of mice scruffed by the traditional method have detected severe bradyarrhythmias during immobilization which persist for several minutes after restraint. These effects on the heart rate and rhythm were not seen with the three-finger method. The three-finger restraint is achieved by gripping the skin fold over the scruff with the thumb and middle finger, then substituting the middle finger with the index finger, thus creating a transverse skin fold along the neck. The transverse fold across the neck removes the pressure from the throat area. A short video demonstrating this restraint method may be found on the Norecopa website.
Refined Mouse Handling
Research has demonstrated that picking mice up by the tail induces aversion and high anxiety levels. Two refined and non-aversive handling methods can be used instead; tunnel handling and cupping.
Tunnel handling involves guiding a mouse into a tunnel which is then used to lift them out of the cage. The mouse can be tipped out of the tunnel backwards onto the wire lid, held by the base of tail and/or scruffed for procedures as needed. This method is best used for wild/jumpy/aggressive mice and it is easier to learn for less experienced handlers.
Cupping involves picking mice up and out of the cage using cupped hands. This method doesn’t require any equipment, but it does require some training of the mice. This methods is best used for pups, young weanlings, older or larger mice, and mice with delicate implants.
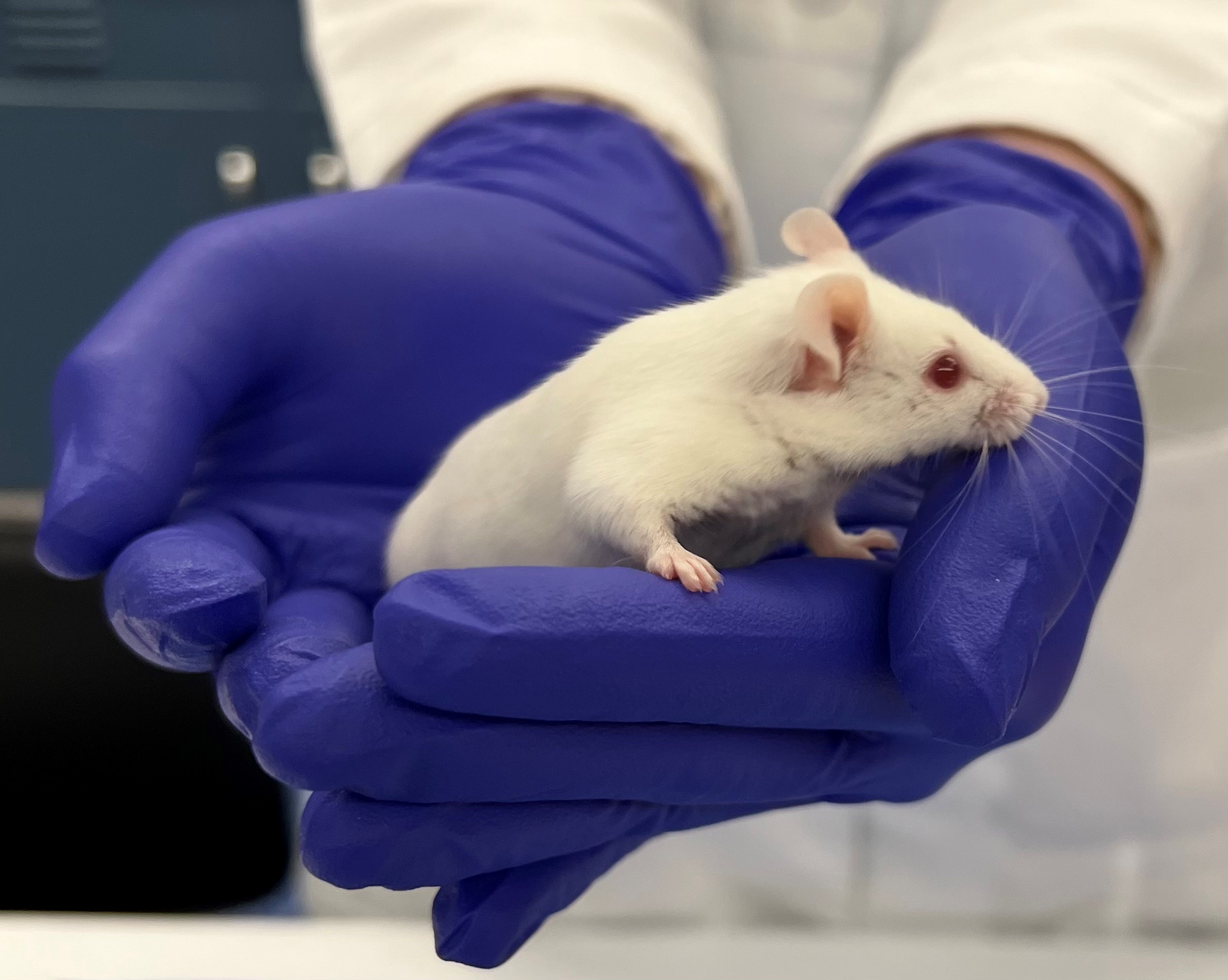
Strong scientific evidence indicates that refined handling, like tunnel handling or cupping, is beneficial to animal welfare and scientific outcomes. For example, mice handled with a tunnel show improved performance in behavioral tests compared to mice grasped by the tail. In addition to reduced anxiety levels, increased voluntary interaction, and improved physiological parameters, non-aversive handling has been shown to improve breeding performance.
Mice quickly habituate to tunnel handling and after a few short training sessions to cupping. Importantly, the positive impacts of non-aversive capture remain even if mice are subsequently administered injections, have blood drawn, or undergo gas anesthesia. For more detailed information on how to habituate your mice to tunnel handling or cupping, you can review the free e-learning Refined mouse handling course developed by the NC3Rs in collaboration with The North American 3Rs Collaborative. For additional video tutorials and FAQs please see the NC3Rs website.
If you would like hands on practice or guidance on how to implement tunnel or cupped handling, please get in touch with ARP veterinary staff.
Rat Handling and Tickling
Rats experience stress during handling, especially if they are picked up by the tail. Gentle handling techniques and handling tunnels can be used to transfer rats between cages and help accustom animals to being picked up from the cage. Rat tickling, also known as heterospecific play, is another method for habituating rats to routine handling. It is also a form of social enrichment that makes handling easier and improves rat welfare. This playful handling technique mimics aspects of juvenile rat rough-and-tumble play by using a human hand to tickle the rat. It reduces anxiety towards the handler and new environments. Additionally, tickling rats before a stressful procedure, such as an injection or blood sampling, mitigates the aversiveness of the procedure and makes rats easier to handle. Research has shown that only 15 seconds of tickling for 3 days can be effective! The step-by-step technique is demonstrated in this flyer and this video. For extensive information on how to tickle a rat properly an online Rat Tickling Certification course is also available. The course reviews the rationale behind rat tickling and includes a detailed pictorial/video instruction on the hands-on technique, guidance on implementation, how to assess a rat’s response to the technique and a series of frequently asked questions. Additional information about this handling method can also be found on the North American 3Rs Collaborative.
Rat Restraint Techniques
Various methods may be used to restrain a rat for procedures. This video presentation demonstrates some common techniques for rat restraint.
Rodent Identification Methods
Ear Notching or punching is a simple, relatively low impact method of identification for mice and rats. Common ear numbering systems for use in rodent colonies and individual cages are available online. A published study has indicated that mice show transient increases in physiologic and behavioral stress responses to ear notching but no significant behavioral indices of pain. This video from the Imperial College of London shows how to perform ear notching in mice. Often, mice are notched at weaning and the tissue obtained from the process used for genotyping. Various types of ear punches may be purchased from laboratory suppliers. All punch tools should be disinfected prior to each use. Rusty or dulled punch tools should be discarded.
Metal or plastic ear tags may be applied to the ear flap of mice and rats and used for identification purposes. Ear tags are usually applied at weaning and each tag has a unique number. Mice must be restrained (i.e., handling stress) to read the number on the tag and misreading of tag numbers is common (up to 25% of the time in one study). If tags are not applied correctly, they can fall out or lead to pain, infection and inflammation of the ear flap. All of these issues represent potentially serious concerns that may influence the scientific integrity of studies. A variety of ear tag types and sizes are available from laboratory suppliers. Tags are applied using an applicator specific for the tag type.
Various tattooing systems are available for mice and rats. Tattoos may be placed on the tail, toe or ear flap. Tattooing of the tail or toe is the preferred method for identifying neonatal mice. Tattoos are permanent but often require anesthesia during application, may require restraint for reading and can become difficult to read over time.
Marking pens may be used for temporary identification of mice and rats. Depending on the color of the rodent, either fur over the back or the tail may be marked. The mark will be readable for a few days. Specialized animal markers are available in many colors although any non-toxic, permanent marker may be used.
Microchip transponders are encrypted with a unique, non-replicable number and may be implanted subcutaneously between the scapulae for permanent identification of individual animals. The chips are read using a hand-held scanner. The rodent must be anesthetized for chip implantation using aseptic technique. Various rodent microchips and applicators are available from laboratory suppliers.
