CATS IRB Upgrade: External IRB Changes
Need more information? View a 10-minute recording of our webinar on the External IRB Changes.
Current/Existing Studies Relying on an External IRB
- Good news: all external IRB studies will be in one workspace moving forward!
- Current External IRB studies will be merged into one workspace
- Current studies will be identified by Site number after upgrade
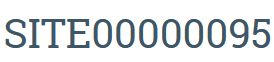
- All study materials will be transferred to and accessible through the Site workspace
- The existing Study # and space will now appear as "Discarded"
- An automatic notification in the History log will note that the Study was discarded--this just refers to the Study #, all materials will be in the Site workspace
- Current studies will be identified by Site number after upgrade
New Studies Relying on an External IRB
- New external IRB studies will be in one workspace, identified by Study #
- A "Site" number will only remain for migrated studies
New Activity for External IRB Studies
 New activity: Update Study Details
New activity: Update Study Details
- "Update Study Details" replaces "Edit Study" -- this means that some study changes no longer require a Site Modification
- When you click "Update Study Details," you will summarize the updates and then be able to make changes to certain pages that do NOT impact insitutional considerations, including Basic Information, Study Funding Sources, Study Scope, and Study-Related Documents
- A Site Modifucation is still required for changes that impact institutional requirements, such as ancillary reviews, institutional consent language, institutional policies, and state law (hint: anything that is referenced in HRP-880 requires a Site Mod!)
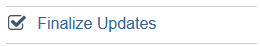
- After Save and Exit, study team must click "Finalize Updates" to finish updating study details

Smartform Changes for External IRB 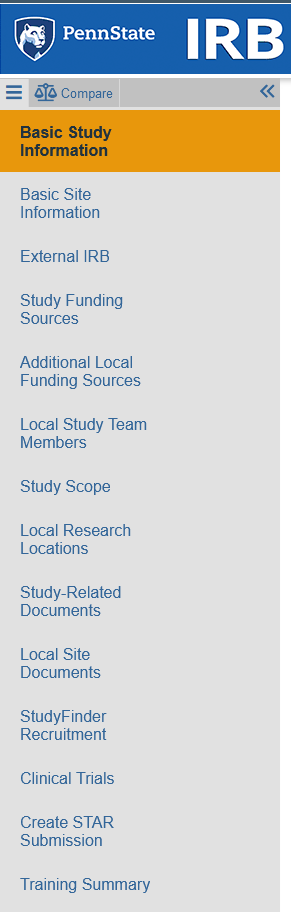
- Basic Information Page
- New Question asks for Lead PI, which may be different from Local PI
- New section "Basic Local Site Information" asks for brief description of activities performed at site
- Process change: HRP-880 and HRP-598 should no longer be attached on this first page, it should be attached to the Local Site Documents page
- New page: Additional Local Funding Sources
- Different from "Study Funding Sources," which is overall project sponsor
- Use this new page to add both the primary source AND funding for the local site (e.g., overall project sponsor plus other institution that is subcontracting to Penn State, internal department funds)
- Local Site Documents
- Attach HRP-880 and HRP-598 here!
- Submissions that were previously approved do not need to relocate these documents.
