Single IRBs and IRB Authorization Agreements
CATS IRB was upgraded successfully in June 2023 with a significant (positive!) process change for External IRB users. Learn more:
- Learn about External IRB Upgrade changes
- View updated Single IRB job aids in the Help Center
- Overview of all upgrade changes
- Watch a 5-minute video about the upgrade
Effective January 25, 2018, the NIH has mandated that all domestic sites participating in a non-exempt, multi-site research study (where study activities outlined in a single protocol are carried out at multiple institutions) use a single IRB (sIRB).
Effective January 20, 2020, OHRP requires a sIRB for all domestic sites participating in a non-exempt, cooperative research study (two or more institutions coordinate to complete a portion of the research outlined in a specific protocol).
Authorization Agreements
An authorization agreement refers to the formal written agreement that documents respective authorities, roles, responsibilities, and communication between an institution/organization serving as the IRB of Record (Reviewing IRB) and the institution relying on that IRB (Relying IRB). This term includes: reliance agreement, cooperative agreement, master services agreement (MSA), master joint agreement (MJA), or umbrella agreement.
Researchers seeking to rely on the IRB of another institution or have Penn State's IRB serve as the reviewing IRB for another institution must have an IRB authorization agreement. These agreements are executed between a Reviewing IRB and one or more Relying Institutions and delineate the roles and responsibilities of the involved parties. The agreements can be for a single research study or for multiple studies (e.g., a master reliance agreement). The authorization agreement is negotiated and finalized by the Penn State IRB and reviewed by Penn State’s legal counsel, as needed.
Authorizations agreements do not replace the need for IRB approval, and all human subjects research protocols still require a submission in CATS IRB, regardless of who may serve as the reviewing IRB. Even with an authorization agreement, researchers must still obtain IRB approval from the reviewing IRB and an acknowledgement of that approval from the HRPP before beginning any study activities and before funds can be released. The Penn State principal investigator remains responsible for ensuring all of Penn State’s institutional requirements are met before beginning the research and throughout the course of the research activities.
Note: Penn State is a member of the SMART IRB Master Common Reciprocal Institutional Review Board Authorization Agreement and has Master Service Agreements in place with multiple commercial IRBs (e.g., WCG, Advarra). If an agreement outside of these already executed reliance agreements is required, it is not uncommon for new authorization agreements to take months because of the various institutional offices that must be involved. Researchers are advised to keep this in mind when considering a reliance request.
sIRB Review Process
sIRB review is more than just an IRB review. It includes other components, such as those depicted in the image below.
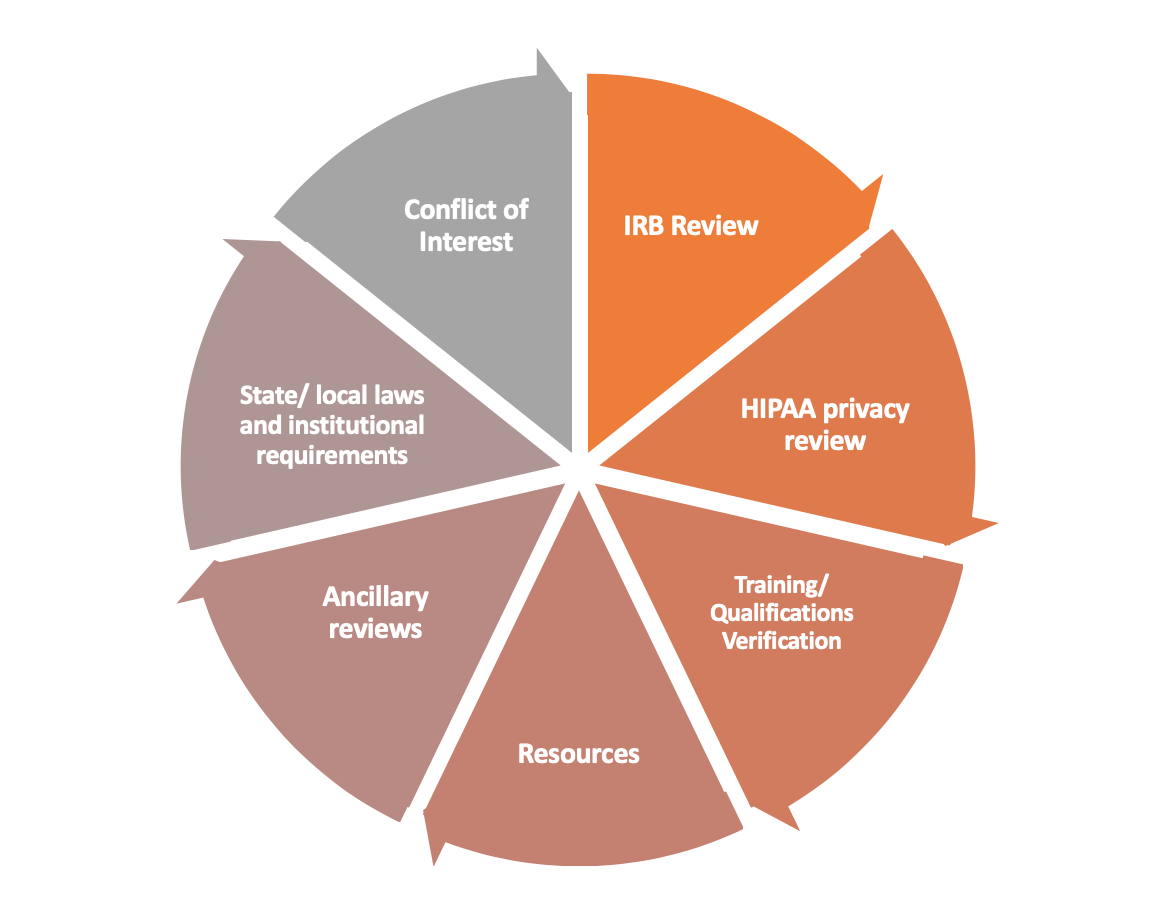
sIRB vs Local Review
The image below depicts the different responsibilities of the sIRB compared to the local institution.
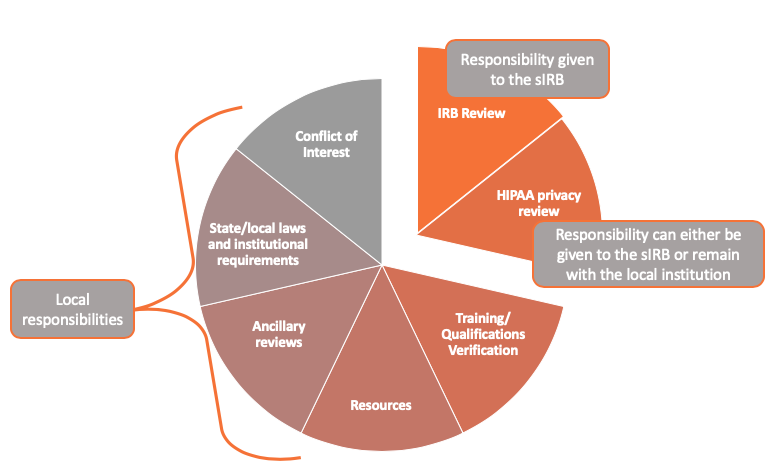
Multi-Site Research
Multi-site studies use the same research procedures (i.e., protocol) outlined in a single protocol that is carried out at multiple institutions.
Examples of Multi-site Research
- Protocols implemented at multiple locations that address the same research questions, involve the same methodologies, and evaluate the same outcomes, such as clinical trials
- Sites that are accruing research participants for studies that are identical except for variations due to local context consideration
Cooperative Research
Cooperative or collaborative research is where two or more institutions coordinate to complete a portion of the research outlined in a protocol at their respective locations.
Examples of Cooperative Research
- A study where one institution gathers the data and another institution analyzes the data
- Protocols that are split between institutions, where one institution may collect a portion of the data, and another institution collects a different portion of the data
Note that the Single IRB requirement only applies to institutions engaged in non-exempt human subject research. For guidance determining if your involvement qualifies as "institutional engagement" in human subjects research, refer to the video provided by the Office for Human Research Protections.
Policies regarding willingness to serve as a Reviewing IRB are specific to each institution. To request that an institution’s IRB serve in this capacity, work with the IRB at that institution (and the principal investigator at that institution, if applicable) to confirm their policy before proceeding. The Penn State IRB makes the final determination regarding whether to be a Reviewing IRB or a Relying IRB when Penn State is engaged in the human subject research study in question.
For questions about Penn State HRPP reliance processes, please contact:
- Rafael Santos, Assistant Director, Reliance & Regulatory Support: rms7210@psu.edu
- Kathleen Lubbers, Reliance Coordinator: kml15@psu.edu
You can also schedule a meeting via Bookings.
Request to Rely (R2R) on an External IRB
5-step R2R at the Penn State HRPP
Investigators should be aware that reliance arrangements frequently require additional work on the part of the local study team, including submission to the Penn State HRPP and the external IRB, as well as managing communication between these offices. For minimal risk research, review times for external IRB protocols can exceed review times for minimal risk projects approved by a local IRB. See the ‘SMART IRB Relying on an External IRB: FAQs for Research Teams’ and the ‘SMART IRB Relying Investigator Checklist’ for additional details about local study team responsibilities.
The section entitled, ‘Reliance on an External IRB for Review of Human Subject Research Studies’ in the Investigator Manual (HRP-103) provides the criteria that Penn State’s IRB considers when deciding to cede review to an external IRB. Typically, we require that the Reviewing IRB is part of an AAHRPP accredited institution or organization. See the AAHRPP website for a list of accredited institutions/organizations.
NOTE: The Penn State HRPP will only enter into reliance arrangements when our site is engaged in non-exempt human subject research. See "Worksheet – Exemption Determination (HRP-312)" within the CATS library to assess whether the activities carried out by our site meets any of the listed criteria. If so, local IRB review should be sought instead.
Investigators requesting a reliance determination should complete a Reliance Request Form. This form must be completed PRIOR to a submission in CATS IRB.
Penn State’s HRPP CANNOT guarantee their willingness to support the use of the sIRB without the submission of this form. If an institution is listed under the "External IRB" list in CATS IRB, there is still a need to complete the Reliance Request Form and confirm the details related to the authorization agreement that will be used. Determinations are made on a case-by-case basis. Penn State will not enter into authorization agreements for research determined to be exempt.
If you are not ready to submit a protocol, but are submitting a grant proposal that includes a single IRB plan, the HRPP has issued a statement confirming our understanding of the NIH single IRB policy and agreeing to rely on sIRBs that meet certain criteria (e.g., using SMART IRB Agreement). The full statement is available for download and may be included in grant applications.
If the Reviewing IRB is requiring a specific letter of support be signed for a grant proposal, or the criteria outlined in the attached statement are not met, please submit the request via the Reliance Request Form at least two weeks before the grant submission deadline.
A CATS submission should only occur after the Penn State HRPP has agreed to the reliance arrangement proposed within STEP TWO.
All human subject research being reviewed by an external IRB, through either a master reliance agreement with a commercial IRB, the SMART IRB Reliance Agreement, or through study-specific reliance agreements with other institutions, must submit an external IRB submission to the HRPP office through CATS for registration and review of local requirements (e.g. conflict of interest, education requirements, state law, institutional policy, ancillary office review). The external IRB submission will be reviewed and returned to you with instruction to submit to the reviewing IRB. Any documents required by the Reviewing IRB should be included with your submission (e.g. local context forms, reliance documents requiring IRB/institutional signatures).
Visit the CATS IRB Help Center to review the "External IRB Submissions" job aid for more information about submitting an external IRB submission to the Penn State HRPP.
For federally funded research, the external IRB submission should only be submitted to the Penn State HRPP after the funding agency has agreed to the proposed sIRB plan.
Reliance on an external IRB is specific to the ethical review of the study, as specified under the HHS regulations at 45 CFR Part 46 and/or FDA regulations at 21 CFR Part 56. Questions about how to submit to the external (Reviewing) IRB should be directed to that IRB. The reliance arrangement may involve standard operating procedures (SOPs) that describe with whom and how to communicate with the external IRB. In addition, the external IRB may have its own distinct steps/processes in its submission process.
Reliance requests that do not fall under an existing Master Agreement with Penn State
Penn State institutional language will likely be required in the consent forms approved by the external IRB. This language can be found in HRP-109 - Consent Language Document. This language should match the Penn State template consent language from the following sections as applicable:
- Compensation for Injury
- Compensation for Participation
- Radiation Safety Language (provided after the local Radiation Safety review)
- Authorization to Use and Disclose Information for Research Purposes
See the 'SMART IRB Informed Consent Documents: Inserting Local Context Language' for additional details about the process for review and approval of local consent forms.
Reliance requests that fall under an existing Master Agreement with Penn State
The IRBs listed below have existing master agreements with Penn State and therefore have Penn State standard consent language and local context information on file. Please reach out to the point of contacts listed below for questions related to submitting to one of these IRBs. For questions about Penn State requirements or CATS IRB submissions please reach out the appropriate HRPP staff.
When using WCG:
- Contact information:
- General WCG IRB questions contact our Client Care Center at 855-818-2289 or by e-mail at clientservices@wcgirb.com
- Escalated or Urgent items contact Christopher Gennai at 360-252-2460 or by e-mail at cgennai@wirb.com and indicate that this is a Penn State study
- Links for the WCG IRB content:
- PSU/WCG Webinar Slides
- WCG IRB Connexus Submission portal (log in or register)
- WCG IRB Connexus Resources (guides and videos)
- WCG IRB Guide for Researchers (general working with WCG IRB guide)
When using Advarra:
- What is the process to use Advarra?
- Once you are ready to submit to Advarra, please visit www.cirbi.net
- Select either the investigator application or protocol application, depending on what you wish to submit to Advarra
- CIRBI helpdesk can be reached at Email: cirbi@advarra.com or toll free at 1-866-99CIRBI (1-866-992-4724)
- For any questions on how to submit, please contact institutions@advarra.com
When using NCI CIRB:
- CIRB helpdesk: ncicirbcontact@emmes.com
- NCI IRB website
Final versions of the study documents approved by the external IRB, alongside the external IRB approval letter, should be attached prior to resubmission of the external IRB submission in CATS. Final acknowledgement of the submission cannot occur until all applicable ancillary reviews have been completed. Because ancillary reviews are by definition ancillary to the IRB review, all criteria for setting ancillary reviews is the same regardless of whether an external IRB is being used.
Please note that research activities cannot begin at any Penn State location until acknowledgment of this final submission has been provided, even if the external IRB has provided their review/approval of the protocol.
Post-Approval R2R Requirements
After an Acknowledgement of Reliance on an External IRB is issued, the following changes must be submitted to the IRB Office for review:
- Revised consent forms
- While all revised consent forms must be submitted in CATS, researchers may implement these changes at PSU once they are approved by the Reviewing IRB UNLESS the proposed changes impact local considerations (e.g., ancillary reviews, PSU standard consent language). Changes that impact local considerations must await PSU HRPP acknowledgement prior to their implementation
- Changes in study team members (for a training status check)
- Changes in funding sources
- Changes in the financial interest of study team member(s) including the PI
- Changes that impact institutional requirements (e.g., ancillary reviews, EIM form, HRP-880, HRP-598)
Visit the CATS IRB Help Center to review the "External IRB Submissions" job aid for more information about submitting a modification to the Penn State HRPP for an external IRB submission.
At this time, the PSU HRPP does not require Continuing Review (CR) submissions or documentation of a CR for External IRB studies. Please consult with the External IRB for CR submission requirements.
Request to Serve (R2S) as the Reviewing IRB
4-step R2S at the Penn State HRPP.
Investigators should be aware that projects designation Penn State as the reviewing IRB for external sites frequently require additional work on the part of the PSU study team, including facilitating communication between the PSU HRPP and the relying sites. The PSU study team must ensure that the relying sites are familiar with Penn State policy, such as RNI reporting timelines. The PSU study team is also responsible for submitting on behalf of all relying sites in CATS, including future continuing reviews (when applicable), modifications, and RNIs. Study teams should consider allocating additional resources for administrative staff support in their grant applications. See the ‘SMART IRB Overall PI Checklist’ for guidance on the responsibilities of the study team for an R2S.
The section entitled, ‘Serving as the Single IRB of Record’ in the Investigator Manual (HRP-103) provides the criteria that Penn State’s IRB considers when deciding to act as the reviewing IRB for a multi-site/collaborative project. Typically, we require that all relying sites are signatories to the SMART IRB Agreement or that all relying sites agree to sign-on prior to submission to the Penn State IRB.
NOTE: The Penn State HRPP will only enter into reliance arrangements when our site is engaged in non-exempt human subject research. See "Worksheet – Exemption Determination (HRP-312)" within the CATS library to assess whether the activities carried out by our site meets any of the listed criteria. If the activities proposed under the grant are eligible for exemption, all sites should seek exempt determinations from their own IRB.
Requests for the PSU IRB to serve as the Reviewing IRB should be submitted well in advance of any funding applications where a single IRB plan is needed. Investigators seeking an R2S should complete a Reliance Request Form. This form must be completed PRIOR to a submission in CATS IRB. Penn State’s HRPP CANNOT guarantee their willingness to act as the Reviewing IRB without the submission of this form.
As per the NIH FAQs, “recipient institutions have the flexibility to develop their own fee structures for single IRB costs”. If the PSU HRPP agrees to serve as the Reviewing IRB and sIRB fees apply, a letter of support will be provided, which provides a direct cost estimate
(included as line item in budget) for the sIRB review fees to be charged to your award over the life of the project. The charges included in this estimate are for the additional costs incurred by providing external IRB review for other sites and are in addition to the indirect costs that cover IRB review for the work carried out by PSU.
The Penn State HRPP currently charges sIRB fees for all multi-site human subject research that designates our office as the reviewing IRB. The fee structure was developed by calculating the number of submissions reviewed by our office in a fiscal year and dividing the total by the budget number during the same time frame. These fees are assessed annually and therefore are subject to change with fluctuations of submission totals and budget numbers.
R2S submissions in CATS are structured differently than projects undergoing local review. If the funding is awarded, the PI and lead coordinator(s) must schedule a meeting with the reliance team in order to discuss submitting your R2S in CATS. Meetings can be scheduled via Bookings. Please see Steps 1 & 2 of the ‘Job Aid for Researchers – Requests to Serve (R2S) IRB Submissions’ located in the Help Center in CATS for guidance in submitting this protocol. R2S submissions require separate STUDY (overall protocol & Penn State) and pSITE (relying site) submissions. As per the Job Aid, you will first submit and obtain approval for the STUDY prior to submitting the pSITEs.
After approval of the STUDY is received, a separate pSITE submission, outlining the local considerations required for that site, will be necessary for all relying sites. Each pSITE will need to be approved by the PSU HRPP prior to the start of activities at each relying site. See Step 3 & 4 of the ‘Job Aid for Researchers – Requests to Serve (R2S) IRB Submissions’ located in the Help Center in CATS for guidance in submitting each pSITE.
Post-Approval R2S Requirements
The PSU study team must ensure that the relying sites are familiar with Penn State policy, such as RNI reporting timelines and for facilitating communication of any local site policies or state laws to the Penn State IRB. The PSU study team is also responsible for submitting on behalf of all relying sites in CATS, including future continuing reviews (when applicable), modifications, and RNIs.
After approval of an R2S is issued, all changes must be submitted and approved by the PSU IRB prior to their implementation except for the following:
- Addition/removal of relying site study team members.
Changes to the template/model documents found in the STUDY should occur prior to submission of the local site version of the documents within each relying pSITE.
Annual continuing reviews may be required if your project is greater than minimal risk OR is FDA regulated. Continuing reviews must be submitted in advance of the expiration date in order to avoid lapses in IRB approval. No research activities may occur if a lapse takes place. When submitting a continuing review for an R2S, please ensure the following:
- Submit the Continuing Review via the STUDY workspace.
- Access each pSITE and utilize the “Report Continuing Review Data” mechanism, found on the left hand side, in order to provide enrollment information for each pSITE.
- Ensure that the totals provided in each pSITE are consistent with the overall totals provided in the STUDY.
SMART IRB Resources
SMART IRB is an initiative developed under an award from the National Center for Advancing Translational Science (NCATS) of the National Institutes of Health (NIH) to support single IRB review in facilitation of multi-site human subjects research.
The Penn State HRPP signed on to the SMART IRB Agreement as a Participating Institution. Using the SMART IRB Agreement as the ‘Reliance Agreement’ is an option if the other Participating Institutions have signed on and also agree to use the SMART IRB Agreement for a given study. A decision to use the SMART IRB agreement is made on a study-by-study basis by each Participating Institution.
Investigators are advised to visit the Resources section of the SMART IRB website for template documents that may be useful in the reliance process, including templates for the sIRB section of a grant application: https://smartirb.org/resources/
Using SMART IRB agreement does NOT replace or negate the internal submission process at Penn State regardless of whether Penn State is the Reviewing IRB or relying on an external IRB. Investigators should never assume that the agreement can or will be used for a given study or convey to others, including a funding agency, that the agreement will be used, without explicit documented agreement from each Participating Institution.
