CATS IRB 2023 Upgrade Highlights
CATS IRB Upgrade
CATS IRB was upgraded successfully on June 26, 2023. Users will notice changes to the interface along with other enhancements; learn more here.

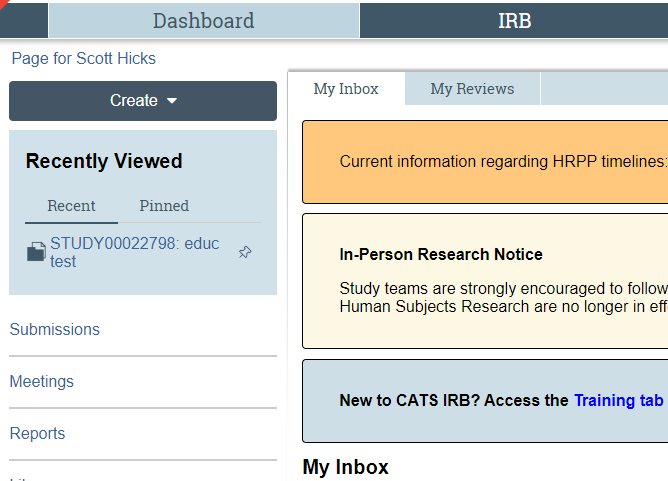
New: Dashboard
The Dashboard is a new feature in CATS IRB. It contains "My Inbox" as well as a new menu: "Recently Viewed." This space allows users to pin frequently visited items so they can more quickly navigate to their studies.
SmartForm Updates
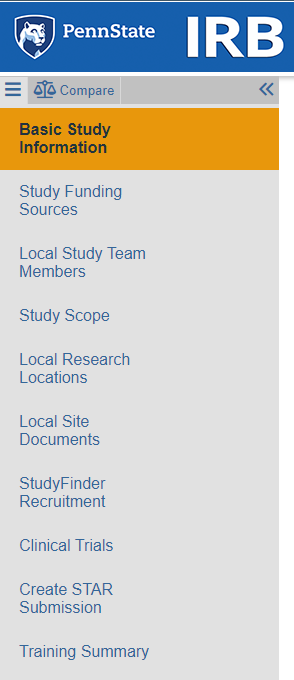
- New: Left navigation pane replaces old "Jump To" menu
- Once your study has been submitted, you can view all of the SmartForm pages at once by scrolling via the new Navigation menu.
- Updates to SmartForm
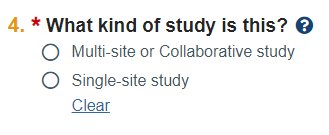
- Small updates/reordering of questions in SmartForm. For example, on the Basic Study Information page, Question 4 has been updated.
- Because of streamlined External IRB workflow, all users will see "Local" in front of pages that refer to activities occuring at Penn State. For example, "Local Research Locations" is a relocated page that was previously part of "Study Scope."
- On the "Drugs" page, you willl need to indicate the type of drug for each drug listed; this will allow the HRPP to accurately fulfill FDA requirements in the event of an inspection.
- Clinical Trials page must be answered for all studies.
- Small updates/reordering of questions in SmartForm. For example, on the Basic Study Information page, Question 4 has been updated.
- "Compare" feature replaces "View Differences."
Other Changes
- Administrative Review, which has been required of certain studies that do not require a Continuing Review, is being removed.
- By ending the use of Administrative Review, Penn State is better aligning with our peers and federal regulations. But just remember that you still need to submit modifications and RNIs as required per the Investigator manual (HRP-103) well as close your study once complete!
- New tab in the IRB Library: “Guidance”
- Consolidates existing HRPP guidance documents previously only available on the website.
- Help Center new feature: users will only see guides related to their roles in the system.
- Watermark: moving forward, consent forms will no longer show "approval end date" in the watermark, just "effective date." Existing forms will be unaffected until the next time they are finalized.
Relying on an External IRB
- External IRB studies will be one workspace moving forward
- Current External IRB studies will be merged into one workspace and will be identified by Site number after upgrade (the existing Study # and space will be deactivated).
- New External IRBs will simply be in one Study space.
- Additional Guidance for External IRBs
- Read an overview of changes for External IRBs
- Attend a 15-minute webinar with an overview of changes: dates and registration.
- Watch a 10-minute video recording of the webinar.
- View updated job aids in the Help Center.
