Modifications to Study Team Members
Study Team Members can be added, deleted, or modified (undergoing a role change) after a study has been approved by the IRB through a modification. Anyone listed on the study can prepare changes to the study; however, only the PI or PI Proxy can submit the change. PIs are responsible for maintaining the study team members listed on the study and ensuring that study team members have met the study training requirements.
For exempt studies, study team changes only need to be submitted if there is a change to the PI or advisor.
When making a change to Study Team Members, verify that new members have completed the required IRB training in CITI. Study Team Member changes may also require a change to other study documents of the submission, such as protocol, consent documents, and recruitment materials. These documents should be revised as part of the modification.
Note re: HRP-509: The use of “Study Team Member Qualification Template” (HRP-509) is only required in limited situations when study team members will perform research procedures requiring special expertise or credentials above and beyond what is typically expected in the course of research conduct and/or clinical care. Additional guidance is provided on the revised HRP-509 form found in the Library in CATS IRB. If the study team is required to have an HRP 509 -Study Team Member Qualification form on file in CATS IRB, this form should be revised and uploaded as an “Update.“ This is in addition to making the required adjustments in CATS IRB as detailed below.
Begin a Modification for Study Team Members
To begin, review the resource on submitting a modification. Once a modification has been started, select the “Study team member information” check box for the modification scope.
Changing the Principle Investigator (PI)
Step one:
- If the Principal Investigator is being changed, select BOTH the “Study team member information” and “Other parts of the study.” The new PI must be updated in the “Basic Information” section and study materials (protocol, consent form, recruitment materials, etc). Until the new PI is approved, the previous PI is still the acting PI and will continue to be listed on the study.
Note: The listed PI and PI proxy are the only individuals able to submit. If required (see Note above), HRP-509 will also need to be revised.
Step two:

- Complete the modification information. List the names of the study team members being added or removed in “Summarize the modifications.”
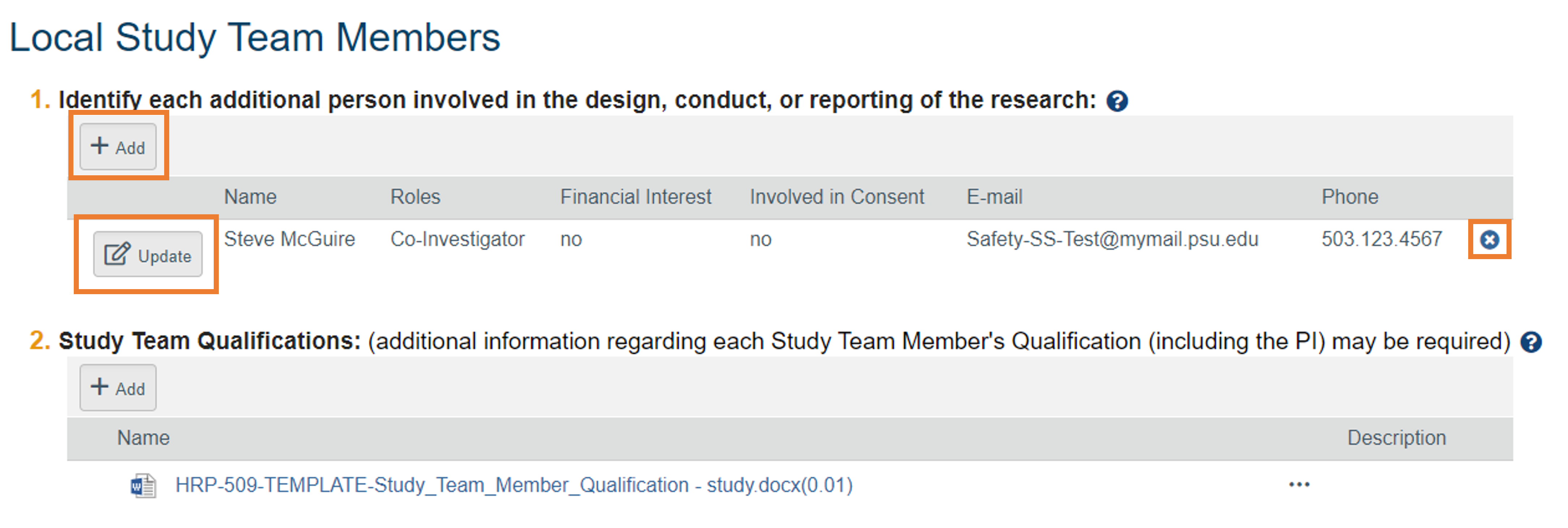
Changing Study Team Members
- To change a study team member’s role, select the “Update” button to the left of their name.
- To add a study team member, select “Add.” New study team members must be approved before engaging in the research.
- To remove a study team member, select the “X” on the right of their name.
- Click “Save and Exit” once all Study Team Member updates are complete. Remember to click the “Submit” button to send the modification to the IRB for review. Steps to submit and confirm submission can be found here.
- If required (see Note above), HRP-509 will also need to be revised and uploaded.