Submit a Modification
Modifications are study changes that should be submitted to the IRB for approval prior to implementing except when an immediate change is needed to eliminate or alleviate an apparent hazard or risk to subjects. Study changes without prior IRB approval must be submitted as Reportable New Information. Example: You wish to change how you’re recruiting participants and you want to add a question to your survey.
Modifications fall into the following categories:
- those that affect the study team membership,
- those that affect other parts of the study,
- or both.
The modification follows a similar review process to an IRB study. Use track changes in Word to document changes to relevant submission materials.
Note: Studies determined to be exempt (not including those that have undergone limited IRB review) only need to submit a modification in certain circumstance : change in Principal Investigator, change in funding, study closure, changes in conflict of interest, and/or proposed changes that might impact the exemption determination (addition of a vulnerable population, change in the way identifiers are recorded so that subjects can be identified, the use of any methods that do not meet the exempt criteria, such as blood draws). With the exception of PI changes, studies determined to be exempt do NOT require modification for study team member changes. See the Investigator Manual for details.
Begin a Modification
1. To begin a modification, navigate to the study in which changes will be made. In CATS IRB, select the “IRB tab” at the top left corner of the screen. Next, select the “Active tab” to display approved studies. Then, select the study in which the changes will be made. For study team changes, refer here for additional steps once the modification has been initiated.
2. Click the “Create Modification/CR” button located in the Next Steps list.

3. Click the “Modification/Update” radio button for changes, including to change team membership.
Note: If there is an active Continuing Review, the radio buttons for Continuing Review and Modification and Continuing Review will not be visible until the current Continuing Review has been approved.

4. When the Modification radio button is selected, the Modification Scope options are visible. Select the appropriate modification type: “Study team member information” or “other parts of the study.”

5. Complete the modification information, which is dependent on the type of modification.
- For question #3, summarize the modification by providing a complete summary of all changes being made (include rationale for changes) and note when the changes impact other documents, such as the consent form. For example, “Due to construction, the interview site will change from 111 Boucke Building to 110 Chambers Building. The consent form detailing the interview site as well as they study protocol are updated to reflect this change in location.”
- For assistance summarizing a modification, view the help text by clicking on the question mark.

Submit a Modification
1. Select update (not ADD) to upload new versions of all documents impacted by the modification.
2. Click “Save and Exit” on the final page once all changes have been made.
Note: Modifications can be edited prior to submitting by selecting the Edit Modification/CR button under Next Steps.
3. Click “Submit” to send the modification to the IRB for review.
Only the PI or the PI Proxy can Submit. If Submit is not selected, the modification will remain in the pre-submission state and will not be submitted. The PI or the PI Proxy will be required to enter their credentials to complete the submission.
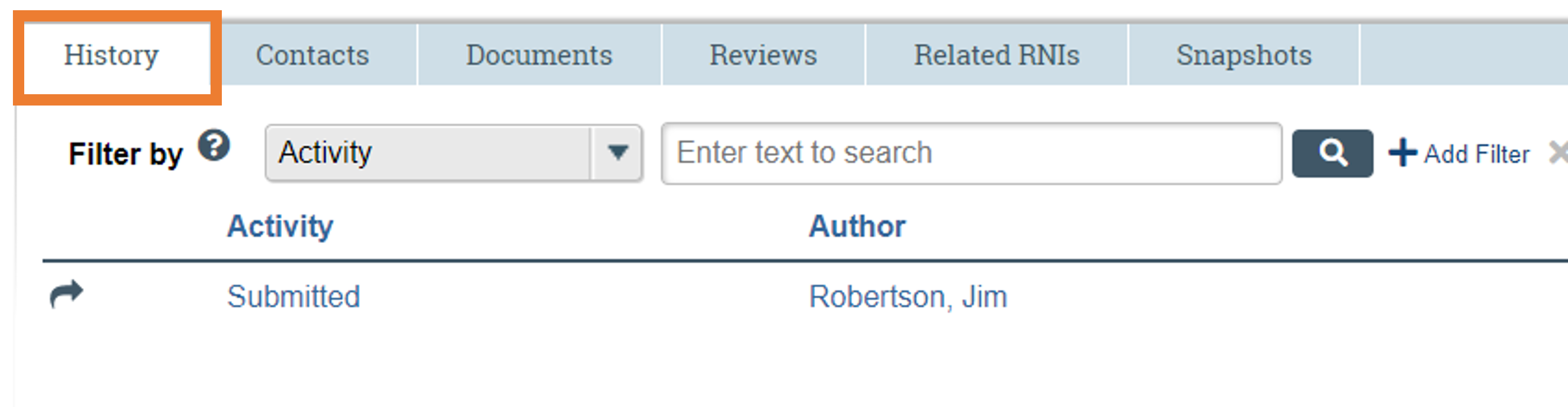
Confirm a Submission
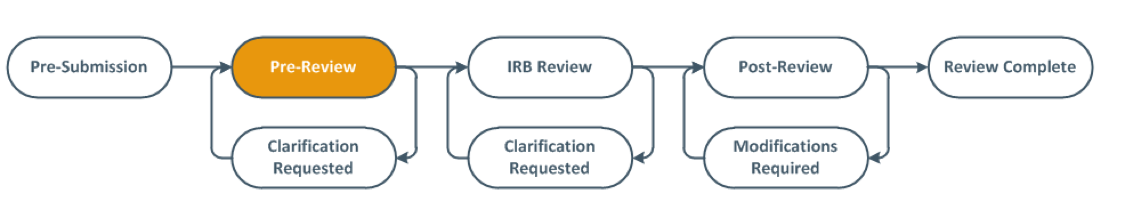
The modification submission is recorded in the History log and the workflow will also change from pre-submission to pre-review. The PI and the Primary Contact will be notified by email when the modification is approved.