Step 3: How to Submit
Submissions to the IRB are made through the online application system, CATS IRB.
Please visit our webpage Create and Submit a Study to the IRB for detailed instructions on how to perform this function in CATS IRB.
Components of an IRB Submission
The following components of a study submission outline the planned research activities for the IRB to review. For assistance accessing these materials, see the IRB resources on the page. Depending on the study, additional materials may be required.
The following study documents must be uploaded with your submission in CATS IRB:
- Study Protocol: The central component of a submission to the IRB. Refer to the Investigator Manual to determine the appropriate Study Protocol for your research. Protocol templates are numbered HRP-591 through HRP-596 in the CATS IRB Library.
- Study Team Member Qualifications: Use template HRP-509, when required, to describe the role and qualifications of each study team member, including education/training, experience conducting assigned procedures, etc. HRP-509 is required if a study team member performs research procedures requiring special expertise or credentials; if a student will be the PI; if individuals will conduct biomedical procedures in a non-clinical setting; or if the research is occuring through an Individual Investigator Agreement.
- Consent Forms and Recruitment Materials: With the exception of studies determined to be exempt, submissions must include a consent form and recruitment materials. Recruitment materials include ads, handouts, scripts, etc. See the Investigator Manual for further guidance related to recruitment. Consent form templates can be found in the CATS IRB Library. Be sure to use the correct template for your campus and the type of study you will conduct. Learn more about consent.
- Grant application: Submissions for research that is externally funded must include any grant application/proposal.
- Other supporting documents: Supporting material(s), such as data collection instruments including but not limited to:
- screening documents
- interview questions
- video/audio/written stimuli to be viewed by participants
- questionnaires
- surveys
Note for in-person research: Study teams are strongly encouraged to follow Best Practices for Conducting In-person Human Subjects Research
Study Submission
Once all components of a submission are complete, the next step is to officially submit to the IRB. As a reminder, all required components of a submission should be uploaded in CATS IRB.
Saving the submission after all materials are uploaded DOES NOT SUBMIT to the IRB. The PI or PI Proxy MUST be the one to submit. Successful submissions will no longer appear in “My Inbox.” Additionally, completed submissions will move from “Pre-Submission” to “Pre-Review” in the study workspace flowchart as pictured below.
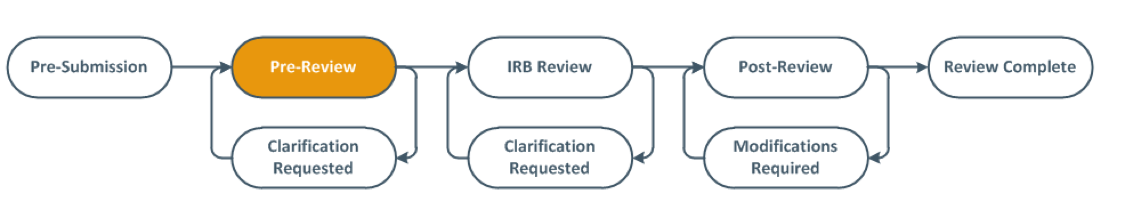
Submissions are assigned to an IRB Analyst based on the first letter of the Principal Investigator’s last name. The IRB Analyst is the primary contact for each study submission. IRB Analysts pre-review of the materials submitted and the determine the IRB category, as well as necessary next steps. Find your IRB Analyst.
What's Next?
Learn about what happens in the review process in Step 4.
